Gut microbes influence how oral drugs are processed and eliminated. Oral drugs, including common non-antibiotics, impact gut microbial balance.
Emerging scientific and clinical evidence from the last decade has reached an indisputable conclusion: the health of our gut microbes (collectively called gut microbiota) impacts our health and wellbeing.
The gut microbiota is constantly interacting and communicating with major anatomic and physiological systems in our body, including the brain and nervous system, digestion and metabolism, immune system, and endocrine (hormonal) system.
Altered and imbalanced gut microbiota has been associated with a dysfunctional gut environment and the development of many diseases, physical, mental and behavioral.
Gut microbes and drug metabolism
Gut microbiota is a key player in oral drug metabolism, influencing the efficacy and toxicity of drugs.
- Before compounds in oral drugs enter blood circulation and reach their target(s), they are subjected to metabolism in our intestine and liver. This pass through process decreases the eventual drug concentration in our body.
- The gut microbiota may metabolize drug compounds before absorption, after outflow from the gut wall into the intestine, or after biliary excretion from the liver. (See figure).
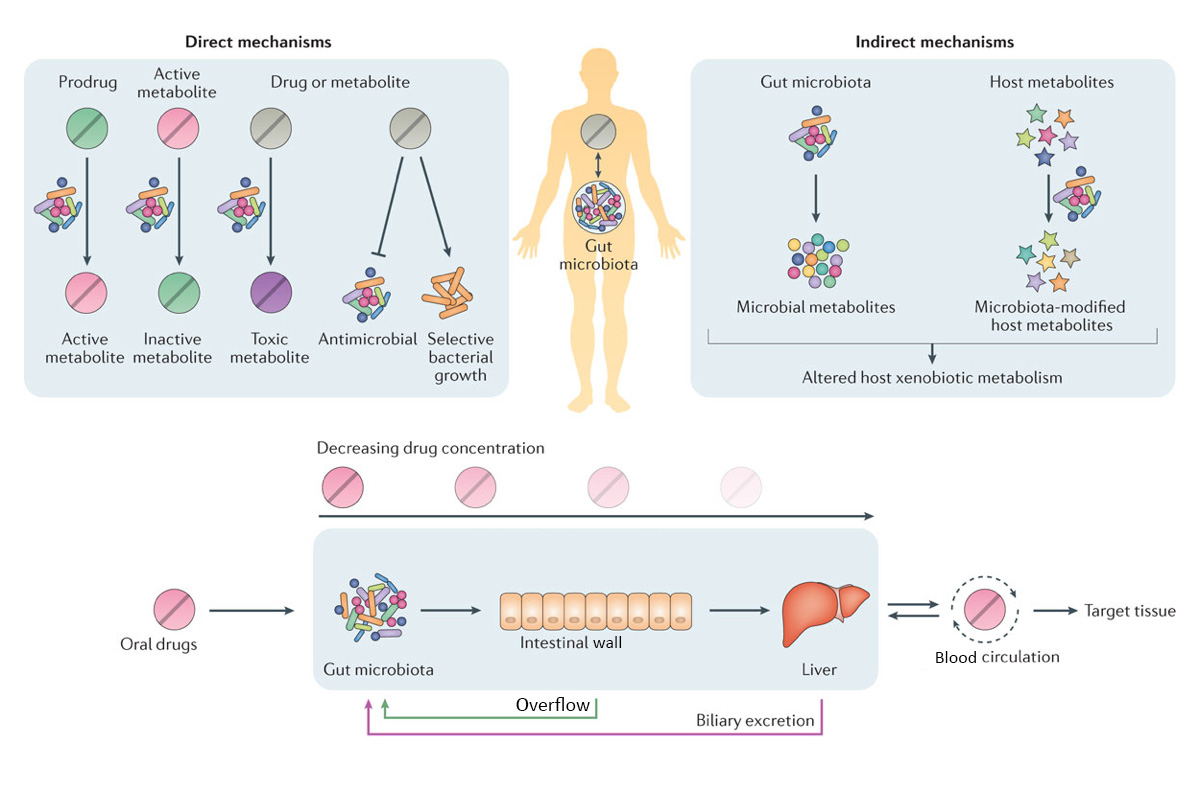
Adapted from The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Peter Spanogiannopoulos, Elizabeth N. Bess, Rachel N. Carmody & Peter J. Turnbaugh. Nature Reviews Microbiology 14, (5)273–287 (2016) doi:10.1038/nrmicro.2016.17
- Gut bacteria can directly metabolize oral drugs into active, inactive, or toxic end products (metabolites).
- Gut bacteria can also influence the drugs through regulation or modification of pathways in our body that are responsible for metabolism, absorption, and transportation. This may be accomplished by the gut bacteria metabolites or by our metabolites that are modified by gut bacteria.
- Host-microbiota interactions can also shape therapeutic outcomes. This may be one reason why people may react differently to the same drug.
For example, metabolism of bile salt by gut microbes may contribute to the effective absorption of simvastatin (cholesterol-lowering drugs). The beneficial effects of the drug tempol (for obesity) are mediated through the gut microbiota.
Compounds in drug may also compete with microbial metabolites for the same metabolism enzymes in the body, affecting the break down and elimination of those drug compounds.
For example, the microbial metabolite p-cresol can affect the metabolism of acetaminophen (paracetamol, trade names include Tylenol and Panadol), one of the most widely used drugs worldwide. Increased p-cresol levels may impede the conversion of the active form of acetaminophen into the inactive forms, hindering the body’s ability to clear acetaminophen.
Studies of drug metabolism, and microbial metabolism in general, will facilitate the discovery of gut microbiota signatures that predict drug outcomes, co-therapies that target specific gut microbes, new drugs that can be harvested from the gut microbiome, and personal medicine strategies.
With advances in gut microbiome research, a more comprehensive view of pharmacology that includes the gut microbes and their interactions as key components of drugs of the future is emerging.
Many drugs affect gut microbial balance
Drugs may shape the composition and functions of the gut microbiota through antibacterial activity, or by promoting/ diminishing specific bacteria. Manny common drugs, not only antibiotics, can upset gut microbial balance impacting health negatively and may even be contributing to antibiotic resistance.
The study
A study, the first that systemically profiled interactions between drugs and individual gut bacteria, reports that 1 in 4 non-antibiotic drugs inhibit the growth of at least one strain of gut microbes.
These drugs include anti-diabetes, proton pump inhibitors (for stomach acid), common non-steroidal anti-inflammatory drugs (NSADs), and antipsychotics.
The 1,079 drugs this study screened cover all main therapeutic classes, not only anti-infection drugs. A total of 40 representative gut bacteria were screened, including 31 commensals that were found in at least 50% of fecal samples of healthy humans from three continents.
Of the 825 human-targeted drugs (target molecules in human cells), 203 (24%) showed anti-commensal activity, inhibiting the growth of beneficial bacteria. Most affected only a few strains, but 40 drugs affected at least 10 strains.
Implications
Different bacteria responded to drugs differently. Overall, species more commonly found across healthy humans were more susceptible, suggesting that key species important for health were more affected.
The researchers also found a previously unnoticed risk – the shift in the composition of our gut bacteria not only contributes to drug side effects, it may also promote antibiotic resistance.
This is because the general resistance mechanisms of microbes to non-antibiotic drugs and to antibiotics seem to largely overlap. But not all resistance are common to both. In some cases, resistance to specific non-antibiotic drugs only trigger sensitivity to specific antibiotics, the researchers explain.
The researchers suggest that their findings provide a reference resource for future research on drug-microbiome interactions, opening new paths for side effect control, personal medicine, and drug repurposing, and broadening the view of antibiotic resistance.