Do gut microbes play a role in Autism Spectrum Disorder, ASD?
The human microbiome is the collection of microbes that exist in and on the human body. The intestine is where the bulk of these organisms are located. Changes and imbalance in the types and/or quantity of gut bacteria (dysbiosis) have been found to impact health, for example in gastrointestinal conditions.
Our gut microbiome also plays a significant role in neurological and immunological, besides metabolic, processes.
A recent study examined the possibility of treating children with autism spectrum disorder (ASD) by modifying the composition of their gut microbiome.
Features of ASD
Autism spectrum disorder refers to developmental disabilities of the brain that are usually apparent in early childhood. The affected children exhibit characteristics that include impairment in social communication and interaction, and repetitive and restricted patterns of activity and behaviour.
Signs of ASD appear in early childhood and usually persist for the person’s lifetime. Their cognitive ability varies. About 30% of ASD persons have an IQ of 70 or less.
The ASD designation is now applied to all individuals who were previously classified into three separate groups, which are no longer diagnosed separately: autistic disorder, pervasive developmental disorder-not otherwise specified (PDD-NOS), and Asperger disorder.
In the former classification, autistic disorder is the most severe form of ASD, PDD-NOS shows some of the symptoms of autistic disorder, and Asperger is a milder form of autism.
The exact cause(s) of ASD are not known. The disorder has a genetic component but no single gene has been found to be involved in all cases of ASD.
The observation that when one member of a pair of identical twins has ASD the other twin does not always have the condition suggests that environmental factors play a role in ASD. Some possibilities include pre-and post-natal exposure to drugs, maternal viral infection and diet. Therapies used thus far to treat the disorder have limited effectiveness.
In the USA, the Centers for Disease Control maintain an Autism and Developmental Disabilities Monitoring (ADDM) Network, which reports on ASD. The 2018 report’s data on the prevalence of ASD for 11 communities that were thoroughly monitored in 2014 indicate that, on average, 1.7% of 8 year old children (i.e. 1 of every 59) were diagnosed as ASD, up from 1.5% in 2012 and 0.7% in 2002. The condition affected 4 times as many males as females.
Why target the gut microbiome
Two lines of observation suggest that the gut microbiome may be involved in the etiology of ASD.
First, in non-ASD persons, dysbiosis of the gut microbiome has been demonstrated to play a role in gastrointestinal conditions such as irritable bowel syndrome (IBS) and others.
In the case of ASD, it has been found that about 40% of the children, more in some studies, have gastrointestinal problems such as constipation, diarrhea, abdominal pain and flatulence, which are significantly more common than in their unaffected siblings.
Some studies also indicate that the severity of ASD is related to the severity of the child’s gastrointestinal problems.
Furthermore, studies comparing the gut microbiome of ASD and non-ASD children report that the ASD children have a less diverse gut microbiome, and lower amounts of certain species of bacteria that they have in common with non-ASD children.
These findings indicate an association between the gut microbiome and ASD but not necessarily a causal relationship.
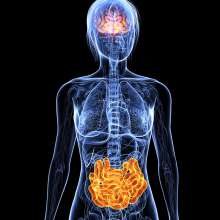
Second, recent studies of the human microbiome and that of other mammals have demonstrated the existence of a gut-brain axis whereby microbiota in the human intestine, via their effects on the immune system and certain chemical substances they produce, interact reciprocally with the brain.
Of specific interest for ASD, studies of germ-free mice show that lack of a gut microbiome results in impaired nervous system functions such as memory and sociability, which can be significantly reversed by introducing microbiota from normal animals into the gut of the germ free animals before they are 4 weeks old. (But transfer at 8 weeks is not successful).
Is it possible that the impaired function of the nervous system of ASD children is likewise related to their gut microbiome?
In a study that suggests support for the gut microbiome as a possible causal factor in the etiology of the disorder, ASD children treated for 8 weeks with the antibiotic vancomycin were found to have significant mitigation of both gastrointestinal and ASD symptoms.
Since vancomycin is a drug that remains in the intestine and is not distributed throughout the body, its direct effects are thought to be strictly on the gut microbiome.
This suggests that the observable effects of vancomycin are due to modification of the composition of the gut microbiota, possibly by destroying bacteria that produce neurotoxic substances. However, within two weeks after treatment ended, the benefits were lost.
Treatment by fecal microbiota transplant (FMT)
If a dysbiotic (imbalanced) gut microbiome plays a role in ASD, then correcting the deficiencies in the microbiome should alleviate the condition.
One method to achieve this is by fecal microbiota transplant (FMT). This is essentially a method used 1,700 years ago in Chinese traditional medicine. Patients were advised to use a solution of human feces for the treatment of various maladies including Wenbing, a condition with symptoms that included severe diarrhea and fever.
Currently, FMT is used to correct the possible deficiencies of the gut microbiota of an afflicted individual by introducing the gut microbiota of a healthy individual.
It has been used successfully in treating individuals with recurrent infection by the bacterial pathogen, Clostridium difficile.
A recent study in 2017 explored the effectiveness of treating ASD children using FMT. The study enlisted 18 children aged 7-16 years old, with mild to severe gastrointestinal problems, who were clinically evaluated for ASD by various standard tests.
The gut microbiota that were to be transplanted were derived from healthy non-ASD individuals whose feces had been screened to rule out any infectious disease, parasites and metabolic disorders.
After preliminary treatment with vancomycin to suppress any pathogenic bacteria in the gut, the ASD study participants were given the stool preparation daily, either orally or rectally, for 7 or 8 weeks. The treatment had no significant adverse effects on the children.
Periodically, the children were evaluated for the microbiome composition in their stools and ASD symptoms. At the end of the treatment period, the children were monitored for a further 8 weeks, the total time of the study being 18 weeks.
Analysis of the gut microbiota at the start of treatment found, as in other studies, that the ASD children had less diversity than the non-ASD children. However, by the end of the treatment period, the diversity had significantly increased and became very similar to the composition of the donors’ microbiota.
Even at 8 weeks after the end of treatment, acquired diversity and similarity remained higher than at the start of treatment.
Evaluation of the children’s gastrointestinal symptoms concluded that there had been substantial improvement with regard to abdominal pain, indigestion, constipation and diarrhea.
In addition, tests that evaluate a variety of ASD behavioural symptoms showed improved scores.
The improvements for both gastrointestinal and ASD symptoms occurred gradually over the 10-week treatment period and, unlike the vancomycin study, were not lost during the 8 weeks following the end of treatment.
Since the microbiota composition of the ASD children became similar to that of the feces donors along with improvement in gastrointestinal and behavioural symptoms, it was concluded that the gut microbiota plays some role in ASD.
Most encouraging is that even 8 weeks after FMT treatment ended, the improvements and the microbiota diversity were sustained, suggesting that the transplanted gut microbiota have colonized the ASD recipients’ intestines.
It will be important to see whether this colonization is long lasting.
The researchers in this study are careful to point out that their work is an open-label study, that is, one which does not randomly select the subjects, does reveal which participants are given the treatment being studied, and does not make use of a placebo control.
A randomized, blinded, placebo controlled study is considered to be the “gold standard” for medical research. Further limitations of the study are the small number of study participants, and the variation in the type and severity of their gastrointestinal and ASD symptoms.
Use of probiotics
Probiotics are strains of bacteria (such as those in yogurt and sour milk) that can be ingested to modify the composition and/or the function of gut microbiome for health benefits.
They have shown some benefits in treating certain gastrointestinal conditions, for example, irritable bowel disease and Crohn’s disease.
If dysbiosis of the gut microbiome is in fact involved in ASD, then probiotics can potentially bring about changes in the ASD microbiota that may improve the condition.
The bacteria used as probiotics are almost always identifiable species that can be cultured and usually mixed in various combinations to produce a probiotic preparation. Such preparations are quite different from FMT preparations, which contain hundreds of different types of bacteria that live and grow in the human gut environment, many of which cannot be cultured using present methods and are known only by their DNA composition.
In one open-label study, a probiotic preparation consisting of 5 living bacterial strains and one dead strain was given for 21 days to 33 ASD individuals, who were monitored for gastrointestinal and ASD symptoms.
Significant improvements in both categories were reported. However as an open-label study, its conclusions are of interest but must be regarded as tentative for reasons mentioned above.
The potential of probiotics as a treatment is also seen in the case report of a 12 year old boy with ASD and abdominal symptoms, who was treated for 4 weeks with VSL#3, a mixture of 10 strains of bacteria.
The severity of the abdominal distress was reduced and examination by a psychologist showed a reduction in overall severity of ASD, which was maintained for almost 10 months after the end of the probiotic treatment.
Other considerations
While these FMT and probiotic studies suggest that modifying the gut microbiome may play a role in ameliorating ASD symptoms, studies of germ free mice raise an important consideration in the use of those methods.
As mentioned above, the aberrant social behavior of germ free animals can only be reversed by introducing gut microbiota from normal mice relatively early after birth.
This suggests that the role of the gut microbiome in normal human neurodevelopment may begin early in life, and once a critical stage is past, modification of the microbiome, by whatever method, may has a more limited positive effect.
In the US, the median age of earliest known ASD diagnosis between 2006 and 2012 was 53 months old. This indicates that if the gut microbiome plays a role in ASD, it does so before about 50 months.
Perhaps treating children younger than 4 years old (48 months) with FMT would give greater relief from the symptoms of ASD?
While there are some safety concerns, a number of studies have treated 1-year-old children with FMT for Clostridium difficile infection and inflammatory bowel disease without adverse reactions, at least in the short term.
How early in life does a possible critical stage occur? This is an interesting question in light of the fact that very recent observations found that even before birth bacteria are associated with the human fetus in the gut, the placenta and amniotic fluid.
In addition to the age of treatment is the question of whether the FMT should use feces of normal children of the same age whose own microbiota is still in the process of stabilizing, or that of older donors.
The studies of fecal microbiome transplant and probiotics described here are exploratory in nature. Further studies with larger numbers of study participants, randomized and blinded, and with placebo controls are needed to demonstrate the effectiveness of both methods.