Few people would consider glass to be an exotic material; on the contrary, we encounter it every time we look at a computer screen or out a window.
However, physicists are still puzzling over the details behind the formation of this ubiquitous substance. The material that we interact with daily in our soda bottles, computer screens, and lenses is actually just one type of glass: silica glass.
Glass state
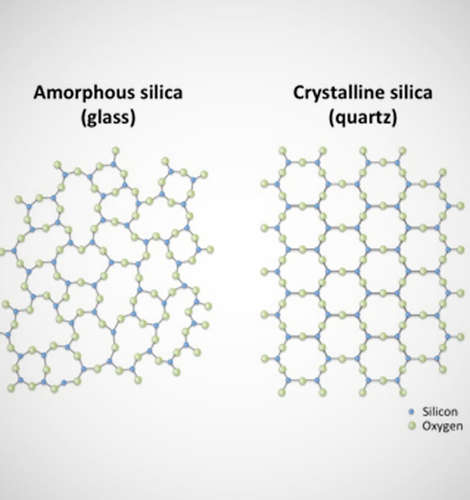
Physicists use the term “glass” much more broadly. To a physicist, ‘glass’ refers to a unique state of matter, and most materials can form a “glassy state” under the right conditions. The glassy state is differentiated from other solid states, such as crystals, by how it appears at the molecular level.
The molecules in a crystalline solid form well-ordered geometric patterns, while the molecules in a glassy solid are disordered.
From a practical point of view, the creation of glass is relatively straightforward. As a liquid is cooled, it reaches a certain temperature, called the freezing point, at which its molecules rearrange themselves from a disordered liquid state into highly ordered crystalline state. A well-known example of this is the freezing of water into ice at 0°C.
However, if a liquid is cooled fast enough, the molecules do not have time to rearrange themselves, and the material will remain liquid-like below the freezing point. At this time, the liquid is “supercooled”.
As the temperature continues to drop, motion of the molecules in the liquid will slow until they come to a stop. At this point, the material exhibits the properties of a solid, while its molecules retain the disorder of a liquid. The material has undergone the glass transition and is in the glassy state.
Transition into glass state
Though people have known how to make glass for hundreds of years, the physics underlying this process remain poorly understood.
Near the glass-transition, the viscosity of a liquid, which can be thought of as the liquid’s ability to “flow”, changes very quickly with temperature. In addition, the liquid displays non-exponential relaxation behavior; this is a (scientific) way of saying that if the liquid is perturbed in some way, such as by compression, it will take longer than expected to return to its initial state.
Theoreticians are working to construct physical models that can explain this behavior. One of the most popular models, referred to as mode-coupling theory, proposes a connection between fast, local interactions (those between a molecule and it’s nearest neighbors) and slower, long-range interactions (those which result in a liquid flow).
In addition, various experimental techniques are being used to further our understanding of the behavior of supercooled liquids at the molecular level. Some of these techniques involve sending acoustic waves through the liquid and observing how it responds, while others involve scattering light off of the liquid and using the patterns to infer information about the motions of the molecules.
Why is it important to understand
A basic understanding of the structure and formation of the glass state will help in the development of new advanced materials. Glassy metal, for example, is generally stronger and more corrosion-resistant than crystalline metal.
However, the structure of glassy metal depends strongly on how it was made; experiments have shown that glassy metal that is flash-cooled may have regions of crystallinity, which disappear if it is allowed to cool at a slower rate.
Relevance in life science
The glassy state is also highly relevant in the life sciences. Foods and biomolecules can be preserved in the glassy state, and it is also believed that some organisms can survive complete dehydration by undergoing a glass transition, which effectively halts all bio-reactions in a cell until it can be re-hydrated.
Though scientists are still a long way from forming a conclusive model of the glass transition, these questions serve as a reminder that sometimes interesting scientific puzzle lie, literally, right in front of our eyes.